Water vapor
Storyboard 
The evaporation of a liquid generates a vapor that can reach a characteristic pressure and concentration known as the saturation point. Typically, the vapors of the liquid are subject to fluctuations, achieving only partial concentrations and pressures that can be characterized based on their degree of saturation. An example of this is relative humidity, which represents the percentage of humidity present in relation to the saturated concentration of water vapor.
ID:(373, 0)
Water Vapor
Image 
The gaseous phase of water corresponds to what is known as water vapor. It is created as water molecules acquire enough kinetic energy to escape from the liquid phase and begin to move through the space above the liquid. Periodically, the molecules in the gaseous state collide with the liquid surface again and are captured, returning to the liquid state.
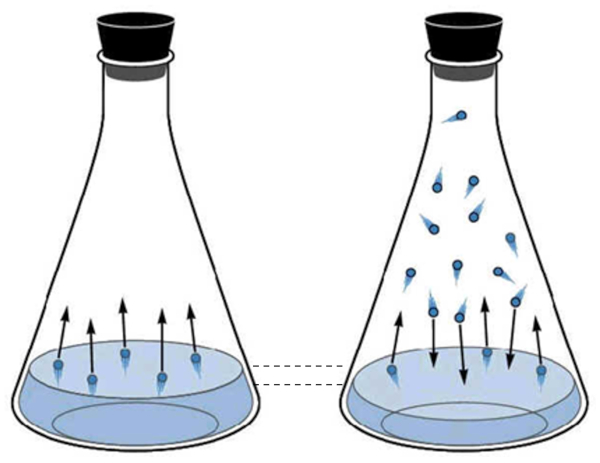
As the number of molecules in the gaseous state increases, so does the number that returns to the liquid. This process continues until an equilibrium is reached between the molecules leaving the liquid and those being reabsorbed. In this situation, it is said that the space above the liquid is saturated.
ID:(1010, 0)
Amount of water vapor
Note 
When the volume variation in phase change ($\Delta V$) changes phase from a liquid to a gas, it can be expressed as:
$\Delta V = V_{\text{gas}} - V_{\text{liquid}}$
Since the volume of the gas is significantly greater than that of the liquid,
$V_{\text{gas}} \gg V_{\text{liquid}}$
we can approximate:
$\Delta V \approx V_{\text{gas}}$
Given that water vapor behaves similarly to an ideal gas, we can state that with the values of the universal gas constant ($R_C$), the number of moles ($n$), the absolute temperature ($T$), and the water vapor pressure unsaturated ($p_v$):
| $ p_v \Delta V = n_v R_C T $ |
Therefore, the volume variation in phase change ($\Delta V$) is:
$\Delta V = \displaystyle\frac{nRT}{p_v}$
ID:(3185, 0)
Pressure saturated water vapor
Quote 
Using the Clausius-Clapeyron equation for the gradient of the pressure ($p$) with respect to the absolute temperature ($T$), which depends on the latent Heat ($L$) and the volume variation in phase change ($\Delta V$):
| $\displaystyle\frac{ dp }{ dT }=\displaystyle\frac{ L }{ \Delta V T }$ |
In the case of the phase change from liquid to gas, we can assume that the change in volume is approximately equal to the volume of the vapor. Therefore, we can employ the ideal gas equation with the number of moles ($n$), the volume ($V$), the universal gas constant ($R_C$), and the pressure ($p$):
| $ p V = n R_C T $ |
Since the Clausius-Clapeyron equation can be written as:
$\displaystyle\frac{dp}{dT}=\displaystyle\frac{L}{n}\displaystyle\frac{p}{R T^2}$
Where the molar Latent Heat ($l_m$) ($l_m = L/n$) corresponds to the change in enthalpy during the phase change h (the energy required to form water), we finally have:
$\displaystyle\frac{dp}{dT}=l_m\displaystyle\frac{p}{RT^2}$
If we integrate this equation between the pressure saturated water vapor ($p_s$) and the pressure at point
$p_s=p_0e^{l_m/RT_0}e^{-l_m/RT}$
If we evaluate this expression with the data at the critical point:
$p_{ref}=p_0e^{l_m/RT_0}$
We finally have:
| $ p_s = p_{ref} e^{- l_m / R_C T }$ |
ID:(15767, 0)
Presión Vapor de Agua
Exercise 
The relationship between the relative humidity ($RH$) with the concentration of water vapor molecules ($c_v$) and ERROR:4952,0 is expressed as:
| $ RH =\displaystyle\frac{ c_v }{ c_s }$ |
and by relating the pressure ($p$) with the molar concentration ($c_m$), the absolute temperature ($T$), and the universal gas constant ($R_C$), we obtain:
| $ p = c_m R_C T $ |
This applies to the vapor pressure of water, where:
$p_v = c_v R T$
and the saturated vapor pressure of water:
$p_s = c_s R T$
resulting in the following equation:
| $ RH =\displaystyle\frac{ p_v }{ p_s }$ |
ID:(15768, 0)
Vapor
Storyboard 
The evaporation of a liquid generates a vapor that can reach a characteristic pressure and concentration known as the saturation point. Typically, the vapors of the liquid are subject to fluctuations, achieving only partial concentrations and pressures that can be characterized based on their degree of saturation. An example of this is relative humidity, which represents the percentage of humidity present in relation to the saturated concentration of water vapor.
Variables
Calculations
Calculations
Equations
Using the Clausius-Clapeyron equation for the gradient of the pressure ($p$) with respect to the absolute temperature ($T$), which depends on the latent Heat ($L$) and the volume variation in phase change ($\Delta V$):
In the case of the phase change from liquid to gas, we can assume that the change in volume is approximately equal to the volume of the vapor. Therefore, we can employ the ideal gas equation with the number of moles ($n$), the volume ($V$), the universal gas constant ($R_C$), and the water vapor pressure unsaturated ($p_v$):
Since the Clausius-Clapeyron equation can be written as:
$\displaystyle\frac{dp}{dT}=\displaystyle\frac{L}{n}\displaystyle\frac{p}{R T^2}$
Where the molar Latent Heat ($l_m$) ($l_m = L/n$) corresponds to the change in enthalpy during the phase change h (the energy required to form water), we finally have:
$\displaystyle\frac{dp}{dT}=l_m\displaystyle\frac{p}{RT^2}$
If we integrate this equation between the pressure saturated water vapor ($p_s$) and the pressure at point
$p_s=p_0e^{l_m/RT_0}e^{-l_m/RT}$
If we evaluate this expression with the data at the critical point:
$p_{ref}=p_0e^{l_m/RT_0}$
We finally have:
The pressure ($p$), the volume ($V$), the absolute temperature ($T$), and the number of moles ($n$) are related through the following physical laws:
• Boyle's law
• Charles's law
• Gay-Lussac's law
• Avogadro's law
These laws can be expressed in a more general form as:
$\displaystyle\frac{pV}{nT}=cte$
This general relationship states that the product of pressure and volume divided by the number of moles and temperature remains constant:
The pressure ($p$), the volume ($V$), the absolute temperature ($T$), and the number of moles ($n$) are related through the following physical laws:
• Boyle's law
• Charles's law
• Gay-Lussac's law
• Avogadro's law
These laws can be expressed in a more general form as:
$\displaystyle\frac{pV}{nT}=cte$
This general relationship states that the product of pressure and volume divided by the number of moles and temperature remains constant:
The relationship between the relative humidity ($RH$) with the concentration of water vapor molecules ($c_v$) and ERROR:4952,0 is expressed as:
and by relating the pressure ($p$) with the molar concentration ($c_m$), the absolute temperature ($T$), and the universal gas constant ($R_C$), we obtain:
This applies to the vapor pressure of water, where:
$p_v = c_v R T$
and the saturated vapor pressure of water:
$p_s = c_s R T$
resulting in the following equation:
When the pressure ($p$) behaves as an ideal gas, satisfying the volume ($V$), the number of moles ($n$), the absolute temperature ($T$), and the universal gas constant ($R_C$), the ideal gas equation:
and the definition of the molar concentration ($c_m$):
lead to the following relationship:
When the pressure ($p$) behaves as an ideal gas, satisfying the volume ($V$), the number of moles ($n$), the absolute temperature ($T$), and the universal gas constant ($R_C$), the ideal gas equation:
and the definition of the molar concentration ($c_m$):
lead to the following relationship:
Examples
Evaporation occurs when particles in a liquid reach its surface and possess enough energy to escape, becoming part of the gas we call vapor. For this to happen, some particles must have sufficient energy to overcome the attractive forces between the molecules in the liquid. This energy is mainly kinetic, so their ability to escape depends on reaching a minimum velocity.
The gaseous phase of water corresponds to what is known as water vapor. It is created as water molecules acquire enough kinetic energy to escape from the liquid phase and begin to move through the space above the liquid. Periodically, the molecules in the gaseous state collide with the liquid surface again and are captured, returning to the liquid state.
As the number of molecules in the gaseous state increases, so does the number that returns to the liquid. This process continues until an equilibrium is reached between the molecules leaving the liquid and those being reabsorbed. In this situation, it is said that the space above the liquid is saturated.
When the volume variation in phase change ($\Delta V$) changes phase from a liquid to a gas, it can be expressed as:
$\Delta V = V_{\text{gas}} - V_{\text{liquid}}$
Since the volume of the gas is significantly greater than that of the liquid,
$V_{\text{gas}} \gg V_{\text{liquid}}$
we can approximate:
$\Delta V \approx V_{\text{gas}}$
Given that water vapor behaves similarly to an ideal gas, we can state that with the values of the universal gas constant ($R_C$), the number of moles ($n$), the absolute temperature ($T$), and the water vapor pressure unsaturated ($p_v$):
Therefore, the volume variation in phase change ($\Delta V$) is:
$\Delta V = \displaystyle\frac{nRT}{p_v}$
Using the Clausius-Clapeyron equation for the gradient of the pressure ($p$) with respect to the absolute temperature ($T$), which depends on the latent Heat ($L$) and the volume variation in phase change ($\Delta V$):
In the case of the phase change from liquid to gas, we can assume that the change in volume is approximately equal to the volume of the vapor. Therefore, we can employ the ideal gas equation with the number of moles ($n$), the volume ($V$), the universal gas constant ($R_C$), and the pressure ($p$):
Since the Clausius-Clapeyron equation can be written as:
$\displaystyle\frac{dp}{dT}=\displaystyle\frac{L}{n}\displaystyle\frac{p}{R T^2}$
Where the molar Latent Heat ($l_m$) ($l_m = L/n$) corresponds to the change in enthalpy during the phase change h (the energy required to form water), we finally have:
$\displaystyle\frac{dp}{dT}=l_m\displaystyle\frac{p}{RT^2}$
If we integrate this equation between the pressure saturated water vapor ($p_s$) and the pressure at point
$p_s=p_0e^{l_m/RT_0}e^{-l_m/RT}$
If we evaluate this expression with the data at the critical point:
$p_{ref}=p_0e^{l_m/RT_0}$
We finally have:
The relationship between the relative humidity ($RH$) with the concentration of water vapor molecules ($c_v$) and ERROR:4952,0 is expressed as:
and by relating the pressure ($p$) with the molar concentration ($c_m$), the absolute temperature ($T$), and the universal gas constant ($R_C$), we obtain:
This applies to the vapor pressure of water, where:
$p_v = c_v R T$
and the saturated vapor pressure of water:
$p_s = c_s R T$
resulting in the following equation:
The evaporated Mass ($\Delta m$) is defined using the latent Heat ($L$) and the phase Change Heat ($\Delta Q$) as follows:
In many cases the latent molar heat is not available but the latent heat that is expressed, for example, in Joules per kilogram (J/Kg). Since the vapor pressure equation works with the latent molar heat we must convert the latent heat into latent molar heat. Since the latter is per mole, it is enough to divide the latent heat
In the case of water, the latent heat of evaporation is of the order of
The change in volume between the material in two different states can be expressed in moles
to obtain a characteristic indicator of the material.
The pressure saturated water vapor ($p_s$) can be calculated using the reference pressure ($p_{ref}$), the universal gas constant ($R_C$), the absolute temperature ($T$) and the molar Latent Heat ($l_m$) according to the following formula:
The pressure ($p$), the volume ($V$), the absolute temperature ($T$), and the number of moles ($n$) are related by the following equation:
where the universal gas constant ($R_C$) has a value of 8.314 J/K mol.
The pressure ($p$), the volume ($V$), the absolute temperature ($T$), and the number of moles ($n$) are related by the following equation:
where the universal gas constant ($R_C$) has a value of 8.314 J/K mol.
The relative humidity ($RH$) can be expressed in terms of the water vapor pressure unsaturated ($p_v$) and the pressure saturated water vapor ($p_s$) as follows:
The pressure ($p$) can be calculated from the molar concentration ($c_m$) using the absolute temperature ($T$), and the universal gas constant ($R_C$) as follows:
The pressure ($p$) can be calculated from the molar concentration ($c_m$) using the absolute temperature ($T$), and the universal gas constant ($R_C$) as follows:
The relationship between the concentration of water vapor molecules ($c_v$) and ERROR:4952,0 is referred to as the relative humidity ($RH$). In other words, when a relative humidity of 100% is reached, the existing concentration will be equal to the saturated concentration.
ID:(373, 0)
