Thermal conduction
Storyboard
The heat transport is composed of two processes that alternate. On the one hand there is the conduction through one medium and on the other the transmission from one medium to another.
In transport through a medium it is composed of the transmission to the medium, the conduction through it and finally the transmission outside the medium. In a more complex medium should be considered multiple conduction is transmissions from one medium to another. In the event that heat is generated or absorbed in one of the means, conduction from said source or sink is considered.
ID:(313, 0)
Heat Flow
Description
If two bodies are at a different temperature, a heat flow starts from the object at a higher temperature than at a lower temperature.
The heat that enters the lower temperature body contributes to raising its temperature.
The heat that leaves the body warmer leads to this body reducing its temperature.
The increase in the temperature of the coldest body and the reduction in the temperature of the warmest leads to a reduction in the temperature difference that in turn reduces the flow between the bodies.
It is a continuous process until the temperatures equalize leading to a total cessation of the heat flow between the two systems.
ID:(203, 0)
Heat conduction mechanism
Image
To understand how heat is conducted, you can think of a body as being represented by chains of springs that oscillate according to their temperature. If one end of the body has a higher temperature, the corresponding oscillation will also be greater. Since neighboring components oscillate to a lesser extent, they will be gradually affected, leading to the slow distribution of the oscillation/temperature along the chain, as depicted below:
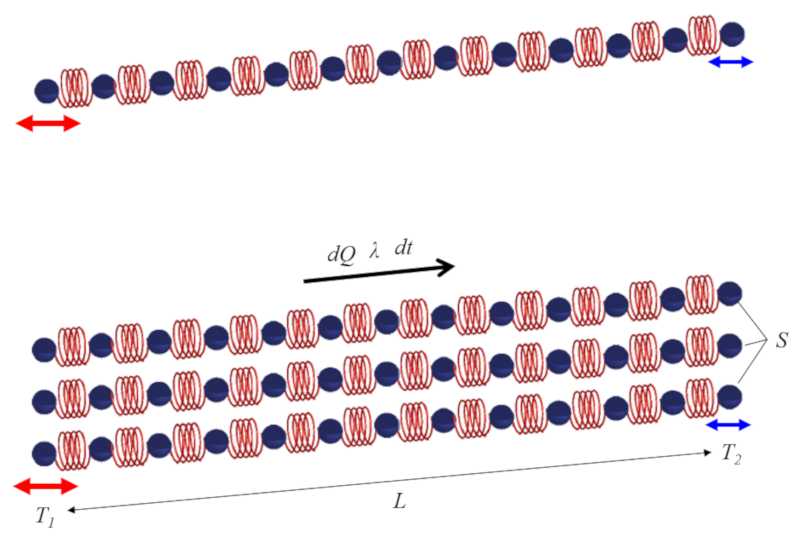
Conduction will be more effective when there are more chains (cross-sectional area) present, and it will be slower as the chains become longer.
ID:(11188, 0)
Heat Conduction
Image
The heat conducted by a
and the conductivity
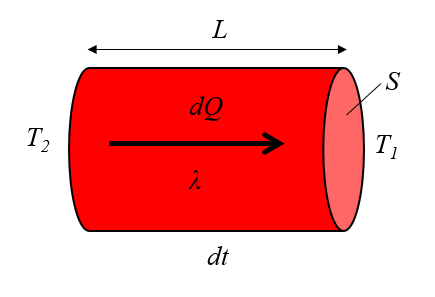
ID:(7718, 0)
Calculation of Heat Conduction
Equation
Heat conduction is the transport of heat along a body or material generated by temperature gradients.
The amount of heat transported
where
The thermal conduction constant is usually expressed in
of conductivity is that of water that is
ID:(3482, 0)
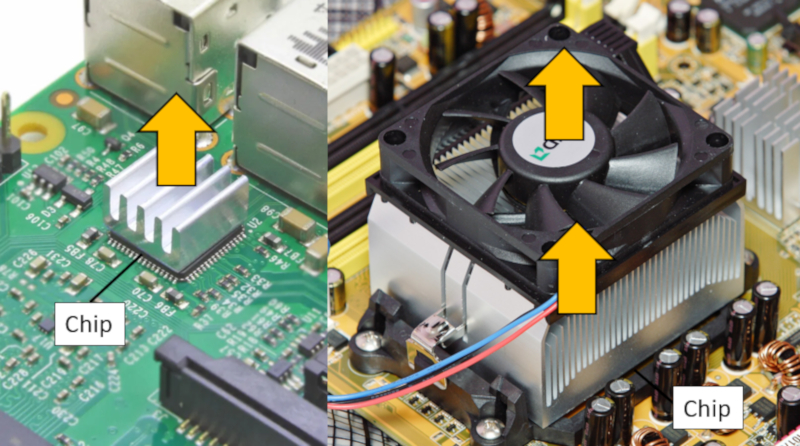
How chip cooling works
Image
Computer chips generate a significant amount of heat and, therefore, need to be cooled. In the case of smaller chips, a high-conductivity metal block is attached to them, creating shapes with large heat exchange surfaces. However, in the case of CPUs (Central Processing Units), this isn't sufficient, and a massive element with a large number of surfaces needs to be added, along with a dedicated fan for active cooling, as depicted in the following image:
ID:(11192, 0)
How a thermos works
Image
The key to a thermos lies in its internal double-walled bottle with a vacuum space that does not conduct heat. Heat transport through radiation, which propagates in a vacuum, is minimized by reflective walls (silver in color), as depicted in the following image:

ID:(11191, 0)
0
Video
Video: Heat transport mechanisms
