Paramagnetismo
Storyboard 
Paramagnetics are materials that under an external magnetic field polarize creating their own magnetic field. However this is not permanent, that is to say when they are removed from the external field they return to a state of magnetic depolarization.
ID:(488, 0)
Magnetization
Definition 
Paramagnetism describes a behavior in which materials can be magnetized based on an applied external magnetic field. In this sense, they do not remain magnetized and lose this property as soon as the external field is removed.
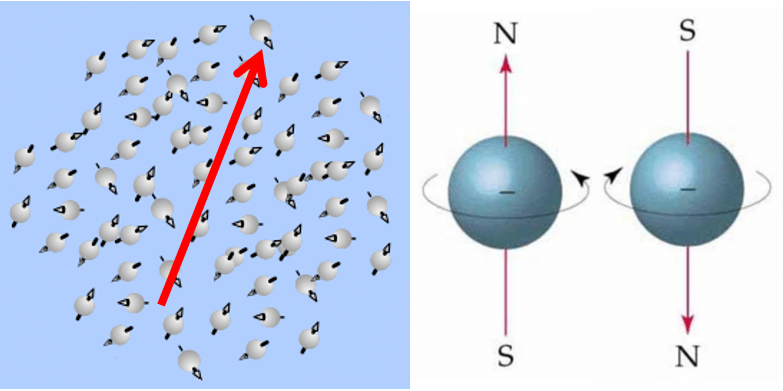
Materials with paramagnetic properties include magnesium, molybdenum, lithium, and tantalum.
ID:(12106, 0)
Paramagnet
Image 
Paramagnetism describes a behavior in which materials can become magnetized in response to an applied external magnetic field, but they do not retain the magnetization when the external magnetic field is removed.
Paramagnetism originates from three types of magnetic moments:
• The magnetic moment of the nucleus (denoted as $\mu_n$)
• The magnetic moment of the electrons (denoted as $\mu_s$)
• The magnetic moment resulting from the motion of electrons in the orbitals (denoted as $\mu_l$)
The first of these magnetic moments is generally much smaller than the other two and is often negligible. The total magnetic moment of the electron ($S$) and orbital ($L$) magnetic moments can be calculated using the formula:
$\mu_{L+S}=\sqrt{4S(S+1)+L(L+1)}\mu_B$
where $\mu_B$ is the Bohr magneton.
ID:(12107, 0)
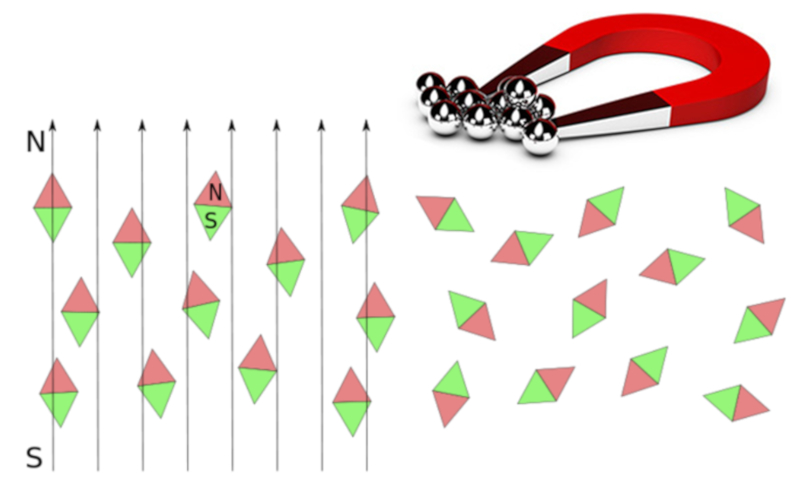
Ferro, para and diamagnetic materials
Note 
Every element can be classified as ferromagnetic, paramagnetic, or diamagnetic with varying levels of magnetization sensitivity. Elements that are ferromagnetic, paramagnetic, or diamagnetic can be identified based on their magnetic properties, and it's important to use the appropriate scales when working with these values.
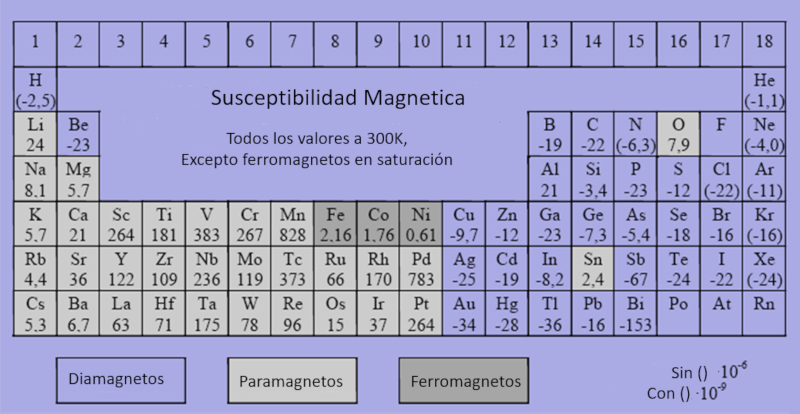
For general data on these classifications, additional resources can be consulted at: Datos.
ID:(12117, 0)
Paramagnetismo
Storyboard 
Paramagnetics are materials that under an external magnetic field polarize creating their own magnetic field. However this is not permanent, that is to say when they are removed from the external field they return to a state of magnetic depolarization.
Variables
Calculations
Calculations
Equations
Examples
Paramagnetism describes a behavior in which materials can be magnetized based on an applied external magnetic field. In this sense, they do not remain magnetized and lose this property as soon as the external field is removed.
Materials with paramagnetic properties include magnesium, molybdenum, lithium, and tantalum.
Paramagnetism describes a behavior in which materials can become magnetized in response to an applied external magnetic field, but they do not retain the magnetization when the external magnetic field is removed.
Paramagnetism originates from three types of magnetic moments:
• The magnetic moment of the nucleus (denoted as $\mu_n$)
• The magnetic moment of the electrons (denoted as $\mu_s$)
• The magnetic moment resulting from the motion of electrons in the orbitals (denoted as $\mu_l$)
The first of these magnetic moments is generally much smaller than the other two and is often negligible. The total magnetic moment of the electron ($S$) and orbital ($L$) magnetic moments can be calculated using the formula:
$\mu_{L+S}=\sqrt{4S(S+1)+L(L+1)}\mu_B$
where $\mu_B$ is the Bohr magneton.
Every element can be classified as ferromagnetic, paramagnetic, or diamagnetic with varying levels of magnetization sensitivity. Elements that are ferromagnetic, paramagnetic, or diamagnetic can be identified based on their magnetic properties, and it's important to use the appropriate scales when working with these values.
For general data on these classifications, additional resources can be consulted at: Datos.
La energ a magn tica de un tomo
en donde
El momento magn tico de un tomo es con
$\gamma\equiv\displaystyle\frac{e}{2m_e}=8.7821\times 10^{10} C/kg$
es el radio girosc pico, con
Si el campo esta en direcci n
con
El momento magn tico se puede expresa con
Si el campo esta orientado en direcci n del eje z se puede reescribir con
$\epsilon=-g\gamma\vec{S}\cdot\vec{H}=-g\gamma HS_z=-g\gamma\hbar Hm$
\\n\\nSi se introduce el magneto de Bohr como\\n\\n
$\mu_B=\gamma\hbar=\displaystyle\frac{e\hbar}{2m_e}=9.2613\times 10^{-24} C m^2/s$
se tiene que la energ a pueden asumir con
Con la definici n de la funci n partici n para un sistema en que los elementos no se sobreponen con
y los niveles de energ a est n definidos con
se puede escribir la funci n partici n para el sistema de
Por analog a el campo magn tico cumple el rol de variable mientras que el momento magn tico la de una fuerza generalizada es con
La suma de una expresi n del tipo\\n\\n
$Z=\displaystyle\sum_{m=-s}^s e^{-\eta m}$
\\n\\nse puede escribir como dos sumas desde 0 a -s y 0 a s restando el elemento 0 que se estar a sumando dos veces\\n\\n
$Z=\displaystyle\sum_{m=-s}^0 e^{-\eta m}+\displaystyle\sum_{m=0}^s e^{-\eta m}-1$
\\n\\nRealizando un cambio de variable (m>-m) en la primera suma se obtiene\\n\\n
$Z=\displaystyle\sum_{m=0}^s e^{\eta m}+\displaystyle\sum_{m=0}^s e^{-\eta m}-1$
\\n\\nDado que las sumas corresponden a series geom tricas finitas se tiene que\\n\\n
$\displaystyle\sum_{m=0}^s e^{\eta m}=\displaystyle\frac{1-e^{(s+1)\eta}}{1-e^{\eta}}$
\\n\\ny\\n\\n
$\displaystyle\sum_{m=0}^s e^{-\eta m}=\displaystyle\frac{1-e^{-(s+1)\eta}}{1-e^{-\eta}}$
\\n\\nlo que da\\n\\n
$Z=\displaystyle\frac{1-e^{(s+1)\eta}}{1-e^{\eta}}+\displaystyle\frac{1-e^{-(s+1)\eta}}{1-e^{-\eta}}-1$
\\n\\nComo\\n\\n
$\displaystyle\frac{1-e^{-(s+1)\eta}}{1-e^{-\eta}}-1=\displaystyle\frac{1-e^{-\eta s}}{e^{\eta}-1}$
\\n\\nla expresi n se puede reescribir como\\n\\n
$Z=\displaystyle\frac{1-e^{(s+1)\eta}}{1-e^{\eta}}+\displaystyle\frac{1-e^{-\eta s}}{e^{\eta}-1}=\displaystyle\frac{e^{(s+1)\eta}-e^{-\eta s}}{e^{\eta}-1}$
\\n\\nSi multiplicamos numerador y denominador por
$Z=\displaystyle\frac{e^{(s+1/2)\eta}-e^{-\eta (s+1/2)}}{e^{\eta/2}-e^{-\eta/2}}$
que se puede escribir con la funci n seno hiperb lico con
Con
la funci n partici n en este caso con
se puede sumar con
Por analog a el campo magn tico cumple el rol de variable mientras que el momento magn tico la de una fuerza generalizada.
Con
se puede definir una temperatura caracter stica con
Como la energ a de un spin en un campo magn tico se puede calcular del momento magn tico
se puede asociar el campo magn tico con la variable generalizada y el momento magn tico con la fuerza generalizada. En tal caso se puede emplear la relaci n entre fuerza generalizada y funci n partici n con
para calcular el momento magn tico medio se puede calcular mediante con
La derivada en el campo magn tico del logaritmo de la funci n con
con
$\displaystyle\frac{\partial\ln Z}{\partial H}=\displaystyle\frac{\partial\ln Z}{\partial\eta}\displaystyle\frac{\partial\eta}{\partial H}=\displaystyle\frac{g\mu_B}{kT}\frac{\partial\ln Z}{\partial \eta}\equiv \displaystyle\frac{g\mu_B}{kT} B_s(\eta)$
donde con
Como el momento de magnetizaci n medio se calcula con
se tiene para la funci n partici n con
se tiene que con
el momento magn tico medio es con
En el limite de altas temperaturas el factor
$coth(x)\sim\displaystyle\frac{1}{x}+\displaystyle\frac{1}{3}x$
y la funci n con
tiende con
El momento magn tico con
que en el limite de altas temperaturas, con
y
se tiende con
ID:(488, 0)
